how do retroviruses such as hiv differ from other viruses?
 Solved: What Is Meant By + And -strand RNA Viruses? How Do... | Chegg.com
Solved: What Is Meant By + And -strand RNA Viruses? How Do... | Chegg.comWarning: The NCBI website requires JavaScript to operate. NCBI Bookshelf. Service of the National Library of Medicine, National Institutes of Health. Baron S, editor. Medical microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Medical microbiology. 4th edition. Chapter 62Humano Retroviruses Miles W. Cloyd. General conceptsHuman Endogenous and Exogenous RetrovirussSimilar to other vertebrate animals, humans possess retrovirus that exist in two forms: as normal genetic elements in their chromosomal DNA (endogenous) retrovirus) and as horizontally transmissible infectious virus containing RNA transmitted from human retrovirus to humans (exogens, for example, HIV and HIV Human T cell leukemia virus, HTLV). Endogenous retrovirus in animals and humans probably evolved from transposable elements, some of them winning the ability to pack into a virion structure, leave the cell and infect another cell. Clinical manifestations In general, endogenous human retrovirus is not pathogens and many of them are not complete viruses. The human genome contains between 100 and 1,000. copies of such viruses and many of them have become pseudogenes or have several flaws. However, some are complete viruses and some genes are transcribing and making virus-coded proteins. The expression of such genes has been found in certain autoimmune diseases in humans like systemic lupis erythematosis and Sjögren syndrome. Endogenous virus gene expression has also been observed in human placentas and reproductive tissues of human beings without any apparent pathology. Human T-Cell leukemia virusHuman T-cell leukemia viruses are transmitted on a horizon from human to human basis (i.e., exogenous viruses) and are associated with the development of some rare diseases. They are believed to have originated from highly related simian virus. Clinical manifestations HTLVs are associated with three presentations: (1) asymptomatic infection (most common); 2) adult T-cell leukemia (LAT) (preadult T-cell leukemia, chronic, chronic acute forms and lymphoma; and (3) tropical paraparesis, neurological disease.StructureHTLVs are spherical particles, l00 nm of diameter, composed of an external lipid bicapa/glucoprotein wrapping that covers an internal protein core. The core contains several copies of reverse transcription (the enzyme that transcribes) RNA to DNA) tied to two identical molecules of united RNA. RNA Codes for internal core proteins (g), external envelope proteins (env), reverse transcriptase (polymerase) (pol), and regulatory proteins (tax, rex). The HTLV-l and HTLV-2 proviral genome lengths are about 9.0 and 8.9 kilobases, respectively. Classification and antigenic types Three types of HTLV are recognized: HTLV-l, HTLV-2 and HTLV-5. HTLVs are classified on the basis of (l) isolation and ability to infect mature T cells and (2) the presence of reverse transcription and internal cross reaction Basic proteins. HTLV-l and HTLV-2 coding regions share about 60% homology. Multiplication After the virion enters the cell, viral reverse transcription transcribes the Viral RNA in DNA, which is integrated into the genome of host cells. Integration Viral DNA can remain inactive or be transcribed into progenie viral RNA and Messenger RNA translated to produce the viral structural and regulatory protein. Progenies virioons are mounted on the cell surface and acquire an envelope for sprouting. PatogenesisHTLV-l and HTLV-2 infected T cells. The viral transactivating protein (tax) becomes about the expression of cell proteins and can lead to uncontrolled control proliferation of target cells. The long incubation period (3O to 40 years) between infection and development of leukemia suggests that events are necessary for leucoemogenesis. The pathogenic mechanisms of the tropical Scenic paraparesis is unknown. Between 2-4% of infected persons develop diseases. Host DefensesHTLV-specific antibodies, cytotoxic and interferon cells have been identified in individuals with persistent infection, but their functions in protection and Patogenesis is not clear. EpidemiologyHTLV-l is endemic in Southwest Japan, the Caribbean Basin, Southeast United States, southern Italy and sub-Saharan Africa. Up to 15% of normal blood donors in the endemic areas of Japan and the Caribbean basin are positive HTLV-l antibodies; in non-endemic areas, less than 1 percent are positive. Data for HTLV-2 are incomplete. HTLVs can be transmitted by blood transfusion of infected donors, sexual contact, and mothers to babies through the breast milk. DiagnosisHTLV diseases are diagnosed by the presence of HTLV specific antibodies and by clinical manifestations. The polymerase chain reaction detects the HTLV genome in infected cells and distinguishes between HTLV-l and HTLV-2 infection. Pre-adult T-cell leukemia is diagnosed based on leucocytosis and abnormal lymphocytes. Acute adult leukemia-linfoma is characterized by presence of pleomorphic neoplastic cells with mature T-cell markers. Chronicle Adult T-cell leukemia is diagnosed on the basis of skin lesions, low levels circulating leukemia cells, and the absence of visceral involvement. Tropical the spastic parparesis is characterized by a meningeal inflammatory process, largely limited to the spinal cord, with progressive weakness in the lower limbs. ControlInfection is avoided by (1) detection of blood products for HTLV antibodies and antibodies (2) Education to prevent sexual contact or exchange needles. Glucocorticoids have had a limited value in the treatment of some cases Tropical paraparesis. Several chemotherapy regimens have been combined tested for adult T cell leukemia with limited success. Human immunodeficiency Virus Clinical manifestationsThe human immunodeficiency virus (HIV) is another exogenous horizontal transmission virus retrovirus and is associated with three presentations: (1) asymptomatic infection; 2) acute infection with symptoms that may include fever, sweating, myalgia or arthralgia, sore throat, lymphadenopathy, nausea, vomiting, diarrhea, headaches and rash; and 3) acquired immunodeficiency syndrome (AIDS), characterized by a progressive immunity deficiency accompanied by a wide range of opportunistic infections, neoplasms and neurological abnormalities, including progressive dementia and peripheral neuropathy. Structure HIV is generally similar to HTLV, although the genome is greater (approximately 10 kilos). As with HTLV, HIV genome codes for basic structural proteins, over protein, reverse transcription, and a set of more regulatory proteins complex than HTLV. Classification and Antigenic TypesThe identification techniques are similar to those used for HTLV. Two antigens types (HIV-L and HIV-2) are distinguished by antibody reactivity to envelope glycoproteins.MultiplicationHIV recognizes host cells by binding the CD4 cell membrane receptor. Multiplication within the host cell is similar to that of HTLV. PatogenesisHIV infects CD4 carrier cells (T4 lymphocytes and monocyte-macrophages). The infection can be latent or chronic at low level. The activation leads to the development of immune dysfunction as a result of direct and indirect killing T4 cells and functional deterioration of viable T4 cells. The macrophages seem to be primary cells in the brain infection. Pathogenic mechanisms Neurological disease is not known. Host defensesPersistent infection stimulates mood-mediated immune responses and cells and cells interferon production, but the role of these responses in preventing pathogenesis or disease progression are not known. EpidemiologyThe HIV infection occurs worldwide and is endemic in Central Africa. United Kingdom States and other developed countries, the infection is mainly found in homosexuals and bisexual men, intravenous drug abusers, hemophiliates, transfusion receptors, sexual partners of infected people, and babies born of infected mothers. Transmission occurs through sexual contact, exposure to contaminated blood or blood and perinatal products. DiagnosisThe HIV infection is determined by the demonstration of HIV-specific antibodies, which usually appear at some time interval after infection. The polymerase chain the reaction detects the HIV genome in infected cells. Detection of p24 core Serum antigen also indicates HIV infection. AIDS is mainly diagnosed base of specific opportunistic infections or cancers coinciding with a T4 cell defect in the absence of other known causes of immunodeficiency. Plus, HIV infections with neuropsychiatric manifestations or severe waste ControlInfection is avoided by detecting blood products for HIV and HIV education prevent sexual contact transmission and needle exchange. Azidothymidine (AZT) and other antiviral agents are used for prophylaxis against progression to disease and treatment. Opportunistic infections and neoplasms are treated individually. Endogenous and Exogenous Retrovirus typesRetrovirus retrovirus, such as virions contain RNA genomes, but the infectious cycle requires copy the RNA genome into the DNA from which the transcription occurs. Therefore, the virus encodes and carries within the virion an enzyme called DNA dependent ARN polymerase or reverse transcription that will transcribe the RNA genome into a double spin Intermediate DNA. This DNA Intermediate is usually covalently integrated into the chromosomal DNA of the cell and therefore becomes a permanent genetic element within That shelter cell. Therefore, there are retrovirus in two forms; as RNA containing virions that spring from a production cell and may infect another cell, and as DNA provirus that can be active or silent. Viruses exist naturally in most vertebrates, as well as some non-vertebrates, and are present in the germline as "normal" genes. Retrovirus is unique in this respect. Human Rights contains a series of retroviral or retroviral sequences within your genome DNA but most of them are silent or have become pseudonyms. However, one or more of these genes can be expressed during normal or pathological conditions, and such "retrovirusendogenous" is known to play roles in natural leukemias and certain immune diseases in non-human situations animals. Endogenous retrovirus is believed to have evolved from transposable elements that are still present in lower forms of life (yes, maze, drosophila). Some retrovirus, however, have evolved to be horizontally transmitted from animals for animals or people to person and these are those that usually cause disease. In man, three "exogenous" horizontal transmission viruses are associated with the disease: HTLV-1 is associated with adult T cell leukemia and tropical spastic paraparesis; HTLV-2 is associated with hairy cell leukemia; and human immunodeficiency virus (HIV) is associated with acquired immunodeficiency Syndrome (AIDS). The retrovirus is classified into three families: Oncoviruss, Lentivirus and Spumavirus. The retrovirus is also classified according to their morphological types in the electronic microscope as type A, type B, type C and Type D. A-type viruses are intracellularly brominated, either in cytoplasm or within endoplasmic reticulum, are not considered infectious, and have an electron lucent core. These are endogenous viruses and some animal species have thousands of copies of these type A viruses in their chromosomal DNA. Their role remains unknown. Type B viruses have an eccentric nucleus and breast tumor viruses They only have this structure. These viruses exist as endogenous and exogenous viruses in some animals and when expressed can cause breast tumors. Type C virus have a central core of electrons, and most of the oncovirus and endogenous The viruses are of this kind. Type D viruses have a rod-shaped core and Lentiviruses They're this guy's. While most endogenous retrovirus are not pathogens, exogens Horizontal transmission viruses are usually. Diseases in animals or human beings are induced by or associated with exogenous horizontal transmission retrovirus, include feline leukemias or sarcomas, chicken leukemias or sarcomas, mouse leukemias sarcomas, equina infectious anemia, bovine leukemia, caprine arthritis-encephalt, human adult T-cell leukemia, human thropic spastic Paraparesis and AIDS. Spumavirus (human foam virus) has not been definitively associated with human disease. Human Cell Leukemia Virus Clinical Manifestations Human infection of T-cell leukemia type 1 (HTLV-l), an exogenous C type oncovirus, results in a spectrum of clinical manifestations ranging from Asymptomatic infection of lymphocyte and neurological disorders (). Most HTLV-l-infected individuals are asymptomatic, with normal white blood cells and differentials Count. However, some asymptomatic individuals may present with moderate lymphocytes and aberrant lymphocytes. About half of these last individuals will progress to a chronic form of adult T cell leukemia (ATL), characterized by small numbers of leukemia cells in peripheral blood, by skin lesions, and lack of involvement of other organs systems. Only 2-4% Infected individuals HTLV-I eventually develop ATL. Approximately 15-20 percent of cases of adult T cell leukemia follow a chronic course. T-cell lymphomas occurs and can involve a variety of organ systems. The most common evil sequela of HTLV-l infection is adult acute T cell leukemia, which follows a aggressive course characterized by malignant polylobular Cells T, hypercalcemia, infiltrated by dermis and epidermis leukemia cells, and immunosuppression which leads to opportunistic infections. Clinical and pathogenesis manifestations of HTLV-1 and HTLV-2 infections. The HTLV-l infection can also result in slow progressive encephalomelopathy called tropical spastic paraparesis or myelopathy associated with HTLV-1. The predominant clinical manifestation is progressive weakness and partial paralysis of the lower extremities. Clinical characteristics can imitate multiple sclerosis. In fact, there is some speculation that a HTLV-related virus is involved in multiple sclerosis. HTLV-l is also reported to be associated with large and malignant granular lymphatic leukemia Hypereosinophilic syndrome. However, these claims have not been substantiated. Two other HTLV, HTLV-2 and HTLV-5 have been identified. The relationship of these virus to human disease is not clear. HTLV-2 has been associated with two cases hair cell leukemia and has been reported in a case of T cells prolymphocytic leukemia and one of T cell chronic lymphocytic leukemia. has been isolated from a patient with T skin cells tac-antigen negative Lymphoma-leukemia (myoid microcosis). HTLV-5 DNA sequences have been found tumor cells of seven other patients with fungoid mycosis. StructureHTLVs are members of the oncovirus family of retrovirus, which are distinguished from other viruses by the presence of reverse transcriptase, enzyme that transcribes RNA in DNA. Most retrovirus is spherical particles, approximately 100 nm in diameter, consisting of an internal protein core surrounded by a glucoprotein envelope embedded in a lipid bicapa. The core contains several copies of reverse transcription linked to two identical RNA molecules united. The DNA copy (provirus) of the genome viral RNA () is flanked at each end by a long terminal repeat (LTR) that contains sequences for the integration of the virus in cellular DNA and the regulation of the expression of the virus. In the viruses that cause chronic leukemias, viral genes encode central proteins (gag), over proteins (env), replication enzymes (pol) and regulator proteins (tax and rex). Some animal retrovirus, instead, are acute transforming. The env gene of these viruses is truncated and replaced by a cellular gene (onc) that causes acute transformation of target cells. HTLV genome (coded proteins). The HTLV envelope consists of two molecular weight glucoproteins approximately 20,000 and 46,000 daltons. Three proteins of mordity (molecular weights) of 9,000, 15,000, 24,000 to 26,000 daltons) constitute the viral core. Different most animal retrovirus, the genome of HTLV codes for two nonstructural proteins, taxes and rex, which are involved in the regulation of the expression HTLV. The tax may also be indirectly involved in the transformation of HTLV lymphocytes. Classification and antigenic types A virus is classified as an HTLV by the presence of reverse transcriptase and by their isolation and infection of mature T cells. In vitro, HTLV infection of T cells result in in their inmortilization (i.e., in continuous proliferation) of cells in the absence of exogenous growth factors). HTLV genomes hybrid only weakly with the genomes of other animal retrovirus. HTLV-1, HTLV-2, and HTLV-5 are currently recognized antigen types. The HTLVs are only remotely related to human immunodeficiency virus (HIV). The HTLVs part internal cross-reaction proteins that differ slightly in molecular weight. The HTLV-1 and HTLV-2 proviral genomes are approximately 9,0 and 8.9 kilos in length, respectively, and have approximately 60% of the general homology. Although the long terminal repeat sequences of these two viruses are quite specific and dissimilar regulatory regions in long terminal repeat are highly Deserved. The other homologous region (over 80 percent) HTLV-l and HTLV-2 genomes is the tax gene. The HTLV-5 genome is less well characterized. HTLV-5 DNA hybrid only weakly to HTLV-1 and not hybrid to HTLV-2. The different HTLVs are distinguished by polymerase chain reaction techniques (PCR) to amplify HTLV and hybridization sequences with specific viral probes. MultiplicationRetrovirus has a common viral replication mode that is based on the function of reverse transcription molecules (). The initial event in retroviral infection is the attachment of the virus to the cell membrane. Specific cell phone HTLV receptors have not yet been identified, but recent experiments have been identified shows that a receptor is encoded by a gene in human chromosome 17. After the union to the cell membrane, the virion enters the cell and is not prepared to release the Viral RNA. The reverse transcriptase, which complexes to viral AR, transcribes the RNA to DNA. The integration associated with virion allows then viral DNA to integrate into the host cell genome. Integrated viral DNA, now called provirus, may remain inactive or be transcribed into viral RNA and the messenger RNA that translates into viral structures and regulations protein. Viral RNA progenie and structural proteins mounted on cellular surface and outbreak of the cell membrane. Since the provirus doubles with the cell DNA during cell replication cycle, cell infection It will persist for the entire life of the clone. Multiplication of human retrovirus. Patogenesis HTLV seem to induce the disease in a fundamentally different way from another retrovirus of oncogen animals. The retrovirus animals of acute transformation causes the malignant transformation by its onc genes that were originally derived of cell growth genes, but they are no longer under cell control and rather They're under the control of the viral promoter. The chronic oncogen animal The retrovirus induce leukemia after a long latent period. Often provirus inserts near a cellular gene. As a result, the cellular gene comes under the control of the viral promoter, thus improving its expression. Therefore, the virally transformed leukemia cells have integrated proviral copies specific sites in cell DNA. In contrast, HTLVs do not have an onc gene, or are integrated in the same site in different tumors (i.e., they show random integration). HTLVs possess two unique genes, taxes and rex, which are necessary for viral replication and efficient transcription of the HTLV genome. The rex gene encodes a molecular weight protein 26,000 to 27,000 daltons located in the core and is required for the accumulation of non-multiplied mRNA gag and for efficient expression of gen gag products. The product of the tax gene is nuclear molecular weight protein 37,000 to 40,000 daltons that transactive viral gene expression. The exact mechanism of tax-induced transactivation is unknown, but it has been suggested that the tax gene product can activate a cell transcription factor that binds to the viral promoter and indirectly increase the expression of the viral gene. Although the expression of the tax gene can lead to cancer in some strains of transgenic mice, the resulting tumors are neurofibromas and do not demonstrate a direct effect of tax about leucoemgénesis. A mechanism by which the product of the HTLV tax gene can play a role in leucoemogenesis is activating cell genes that are involved in T cells growth (). Mitogen-o T cells produce a factor, interleukin-2 (IL-2), which binds to a receptor on the surface of the activated T cells and induces the T cell multiplication. Primary tumor cells of patients infected with HTLV-1 and HTLV-1 T-cell lines that have been converted in vitro with HTLV-1 or HTLV-2 express large number of IL-2 receptors on its surface. In addition, cells transfected with HTLV-1 genes gag-tax release high levels of IL-2 receptors Constitutively. Therefore, it has been hypothesized that the tax gene product is responsible for stimulating the production of IL-2 receptors through the induction of host transcription factors that bind to the IL-2 receptor gene. The research has identified in the IL-2 receptor gene a region that is essential to regulated by taxation of the gene expression. It has also been suggested that the tax protein can transactivate the IL-2 gene itself, although less strongly than the gene of the IL-2 receptor. HTLV can also stimulate T cell growth without actually Infecting the cell. T-cell exposure to HTLV-1 inactivated or partially purified viral proteins can result in mitogenic stimulation and the proliferation of T cells in the absence of exogenous IL-2.Patogenesis of HTLV-induced leukemia. Two infection characteristics indicate that HTLV-induced leucoemogenesis should involve additional pathogens. The first is the long period of incubation between infection with HTLV and the appearance of leukemia cells: it is thought that Leukemia may take several decades to develop. The second feature is the monoclonal nature of malignant cells in adult T-cell leukemia patients. Although HTLV is randomly integrated into the cellular DNA of target T cells, the Adult T-cell leukemia cells in an individual patient originate from a single patient Cell infected by HTLV. Therefore, although HTLV can stimulate polyclonally growth of T cells through fiscal measurement mechanisms or mytogenic stimulation, growth of leukemia cells presumably depends on the fact that it has not yet been identified side events. The HTLV-1 infection also results in the suppression of immunos. In vitro, HTLV-1 infection not only alters the T cell help function causing increased proliferation, but also induces the production of non-specific polyclonal immunoglobulin by B cells regardless of the type of cell that represents the antigen. Infection cytotoxic T cell clones lead to reduction or loss of cytotoxic function. Cells express HTLV-1 has abnormal expression of the main histocompatibility complex (MHC) antigens (cell surface proteins essential for functional interaction of immune cells). Inappropriate expression of these antigens in infected T cells It would prevent or abolish its normal function. In addition, the envelope HTLV-1 and the main complex histocompatibility proteins share certain antigens determinants, so the immune system can be deceived to think that the The HTLV-infected cell is auto and does not need to be removed by the cytotoxic T cell answer. The pathogenesis of the second major clinical manifestation of HTLV-1 infection, Tropical paraparesis is unknown. Several animal retrovirus, especially members of the lentivirus family, may infect the central nerve system and causes chronic neurological diseases in animals. The damage to The central nervous system is generally considered as a result not from a direct point of view neuronal cell infection, but rather the release of toxic factors by HTLV-1 infected cells. Another hypothesis is that the neuronal tissue is damaged by the immune system hosts through an autoimmune mechanism. As an adult T-cell leukemia, spatic tropical paraparesis occurs with very low frequency People infected by HTLV-1 (~1-3%). Even more rare is the occurrence of both diseases in the same individual. Possible explanations for these observations include the existence of different adult T cell leukemia varieties and HTLV-1 tropical parasites induction strains or genetic determinants or host cell factors that contribute to the expression of variable diseases. Host Defenses A characteristic of HTLV infection is its persistence. Once an individual is infected with HTLV, the virus remains in that individual for life. Specific antibodies are produced against a variety of HTLV proteins, including env, gag, and tax. Cytotoxic lymphocytes that recognize viral proteins have also been identified. In patients with tropical spastic paraparesis, HTLV-1 antibody is intratecally synthesized. The role of HTLV-specific antibodies and T cells cytotoxicity in the prevention of new infections or in the progression of diseases is It's not clear. Nor is it clear whether the interferon, produced by HTLV-1-infected cells in culture are beneficial in HTLV infections. HTLV-1 is not crippled by the human serum, although the human complement can smooth another animal retrovirus. The persistence of HTLV in vivo infection indicates that Completely effective immune responses do not occur naturally. EpidemiologyHTLV-l infection occurs, with varying prevalence degrees, in several regions of the world (). The highest prevalence is in South-West Japan, where six endemic areas have been registered identified. The prevalence of anti-HTLV-1 antibodies in the inhabitants of these the areas range from 6 to 37 percent. The Caribbean is another region where HTLV-1 endemic, with a general seropositivity rate of 4%. Between 19 and 48 percent of HTLV-1's relatives - infected individuals in these two endemics areas are seropositive. In both regions, the prevalence of anti-HTLV-1 antibodies increase with age, 2 percent in young children to 30 percent in adults in their stockings. Although there is less data available for Africa, HTLV- 1 infection is also endemic there, with seropositive rates ranging from 4 to 7 percent. HTLV infection is also found in defined populations (e.g., predominantly in intravenous drug users and homosexuals in developed countries like the United States and Italy). It's not clear if the HTLV guy in these populations are HTLV-1 or HTLV-2. Distribution of HTLV. The predominant modes of HTLV infection transmission are by sexual contact, through contaminated blood or blood products, and from mother to child through the breast milk. Studies in Japan have shown that between 48 and 82 percent of recipients Seroconverted seropositive blood. Growing prevalence with age HTLV-1 antibodies is consistent with HTLV-1 sexual transmission. A unique feature of HTLV-1 infection is extremely long incubation period between the initial infection and the appearance of the disease. Incubation period for adults T-cell leukemia can vary from years to years and can be like up to 40 years; incubation period for spastic tropical paraparesis He thought it was shorter but it's still several years. HTLV-1 Antibodies are found in the vast majority of patients with adult T cell leukemia and tropical spasms Paraparesis. However, the incidence of T-cell cancers or tropical spasms Paraparesis in individuals infected by HTLV-1 is extremely low. Studies the incidence of adult T cell leukemia in Japan estimates that less than 0.1% of individuals infected with HTLV-1 develop adult T cell leukemia every year. Adult T-cell leukemia is usually found in individuals over 40 years of age, and mostly in infected individuals like babies. Diagnosis The determination of HTLV infection is usually done on the basis of a positive Anti-HTLV antibody test. Immunosorbene Trial Related to the Enzyme (ELISA) is the most common detection test for the presence of anti-HTLV antibodies. A positive assay is confirmed by direct radioimmunoprecipitation tests or Western Immunoblots. Due to cross-reactivity immunogenic, these tests are not distinguish between HTLV-1 and HTLV-2 infection. Polymerase chain reaction assay, which is based on the amplification of viral DNA segments, is used for distinguish between these two viruses. From the interval between HTLV infection and the appearance of antibodies is not known, the reaction of the polymerase chain can It is also used to diagnose HTLV infection in negative antibodies. The presence of modest leucocytosis with abnormal lymphocytes in a asymptomatic, individual HTLV-infected is indicative of pre-adult T-cell leukemia. Acute T-cell leukemia-linfoma is characterized by presence pleomorphic neoplastic cells with mature T lymphocyte markers. From adulthood T-cell leukemia cells express high levels of Tac antigen (a protein chain that is part of the IL-2 receptor, the diagnosis should include stain for the Tac antigen. Clinical characteristics may include dermalel or epidermal infiltrates, hypercalcemia, bone osteolytic lesions, hepatosplenomegaly and increase susceptibility to opportunistic infections. Chronicles (smoldering) adult T-cell leukemia is diagnosed based on skin lesions, low circulation levels leukemia cells and absence of visceral involvement. Not all cases of adults T-cell leukemia is associated with HTLV-1 infection. Tropical spasm paraparesis is characterized by a meningeal inflammatory process largely limited to the spinal cord, with progressive weakness in the lower sensory extremities and abnormalities. HTLV-1 can be isolated from the blood and cerebrospinal fluid of many patients with tropical spastic paraparesis. HTLV infection and disease control involves three approaches: therapy, education and public health, and vaccination (). Treatment of HTLV-1 infection and its sequel is Extremely difficult. Current treatment regimens for adult T cell leukemia involve combination chemotherapy protocols that do not have significantly increased survival. The elimination of Tac-positive cells by anti-Tac antibodies coupled toxin is being considered as experimental therapy. Glucocorticoids have been used in the treatment of tropical spastic paraparesis, but only limited success. In vitro, azidothymidine (AZT) and dideoxytidine (DDC) inhibit the HTLV-1 infectivity in T4 cells and reduce the growth of infected HTLV-1 cells. The effect of these agents on HTLV in vivo is unknown. HTLV Control. Although there is no treatment for HTLV infection, infected by asymptomatic HTLV people should be routinely examined for evidence of disease progression and advised on ways to prevent the spread of the infection. Methods to Prevent the spread of HTLV infection includes the detection of blood products for antibodies HTLV-1 and HTLV-2, educating people about the prevention of HTLV transmission through sexual intercourse and needle exchange, and advise mothers to refrain from breastfeeding. There is no vaccine against HTLV infection or disease. Development vaccines for retrovirus infections in general have been extremely difficult. Several HTLV-1 experimental vaccines have been designed using the envelope part of the virus like immunogen. A recombinant hybrid HTLV-1 product has been tested in cytomolome monkeys. The monkeys that developed antibodies antibodies anti-HTLV-1 antibodies 20 or more challenge with HTLV-1 production cells. Control monkeys and those with antibody catheters below 20 developed signs of infection between 2 and 6 weeks later inoculation. Rabbits have been successfully vaccinated with vaccine-env virus build. These products have not been tested in humans. Because the development of appropriate therapeutic approaches and vaccines can take many years, the most effective strategy to reduce the number of people with HTLV infection and disease is education. Human Immunodeficiency Virus Clinical ManifestationsIntestinal infection with HIV can lead to acute syndrome with symptoms including fever, sweat, myalgia or arthralgia, sore throat, lymphadenopathy, nausea, vomiting, diarrhea, headaches and rash (). During acute infection there may be a drop in the number of circulating T4 lymphocytes. You don't know what ratio of new HIV infections present with acute syndrome and not Of course these symptoms are due to HIV, Persian. Often other infectious agents are co-transmitted with HIV. After the initial infection, there is a long and long variable asymptomatic period. During this period there may be a slow one, progressive decrease in T4 cell numbers and increase of T8 cells. Some individuals can maintain relatively constant and normal levels of T4 cells during the asymptomatic period. Pathogenesis of HIV infection. Acquired immunodeficiency syndrome (AIDS) is a late manifestation of infection HIV (). AIDS characterized by a marked exhaustion of T4 cells, which leads to an investment of the T4/T8-cel ratio (normally 1.5:1 to 2.0:1) (). Progressive immune deficiency is accompanied by a wide range of opportunistic infections and life-threatening neoplasms, the most common being Pneumocystis carinii pneumonia and Kaposi sarcoma, respectively. Another manifestation of HIV infection is the complex of AIDS dementia (AIDS) encephalopathy). This syndrome is characterized by neurological abnormalities, including progressive dementia and peripheral neuropathy, and may occur in absence of known opportunistic diseases. Clinical manifestations and pathogenesis of HIV infection. Pathogenic mechanisms of HIV infection. Structure The overall structure of HIV is similar to that of the HTLV virus (see above); the virus consists of an external lipid glucoprotein envelope (including over protein gp 120 and gp 41), an internal protein core (protein p15, p17 and p24), a complex viral RNA with reverse transcriptase. The HIV genome approximately 10 kilos, which is greater than HTLV. In addition to the genes of structural mordity, pol and env and regulatory tata (analogous to HTLV g) and rev (analogue to HTLV rex genes), the HIV-l genome contains at least 4 regulatory genes (nef, vif, vpu and vpr). HIV-2 has no sequences for vpu, but encodes a new gene, vpx, which is also found in the ape immunodeficiency virus. Classification and Antigenic TypesHIV is classified as a retrovirus because it contains reverse transcriptase. It is a type D virus in the Lentivirus family. Infection of cultivated T4 cells HIV usually results in cell death. Two major antigens (HIV-L and HIV-2) have been identified and easily distinguished by the differences in the antibody reactivity to the glycoprotein of the envelope. The two types of HIV share approximately 40 percent genetic identity. There's some disagreement about whether they're equally pathogenic. Both seemingly cause AIDS, but some researchers think that HIV-2 is less efficient in causing disease. Individuals isolated from HIV-l and HIV-2 have significant genomic and antigen heterogeneity. The most variable regions of the genome are in the env Gene. This type of variation is also observed in HIV insulated from HIV individuals during the course of their infection. HIV strains often show differences in replicative capacity and cytopathy. The importance of these Variations for the disease process are unclear. Multiplication The first step in HIV infection is the high affinity union of gp 120 about glycoprotein to the CD4 receptor. The CD4 receiver is present in the surface of several types of cells, including T4 cells and monocyto-macrophages. After attachment to the CD4 receptor, HIV multiplication occurs in a similar to that of HTLV (see above). In contrast to HTLV infection, However, the final step of HIV replication often involves the outbreak of the mass cellular surface virion numbers, resulting in cellular lysis. Pathogenesis The high affinity bonding of the HIV glycoprotein envelope to the CD4 receptor is a crucial step in the pathogenesis of HIV, as the main cell expressing CD4 is T4 lymphocyte (often a help cell). The T4 cell plays a central role in all aspects of the function of the immune system, so that the death or deterioration of this cell results in widespread immune dysfunction. There are several potential ways that HIV can damage T4 cells. (1) HIV replication can kill T4 cells as a result of cell membrane destruction by viral proteins. 2) The production of large quantities amounts of viral genetic material and proteins can interfere with normal cells metabolism. 3) HIV can also infect and destroy parent cells that are responsible for the spread of the lymphoid cell pool. In addition to direct cytopathy, HIV infection may indirectly cause T4 cells Death. A mechanism of indirect cell murder may involve autoimmune phenomena in which immunity responses against HIV are directed to non-infected T4 cells or have over-free protein linked to your membrane or present processed Antigens of envelope. In addition, since both the protein of HIV and HIV class II major complex antigens of histocompatibility join the CD4 receptor, their common binding sites can represent antigens that react cross-react. So, anti-HIV antibodies can react with uninfected T4 cells that express class II large complex molecules of histocompatibility. Besides, it's very likely that Anti-HIV inmunitory effects kill many infected cells. HIV-infected individuals often present immune dysfunction before exhaustion of their T4 cells. HIV can induce these functional anomalies by a variety of paths that do not necessarily involve a T4 cell spread infection. Stop. example, by binding to the CD4 receptor, HIV or its protein on can interfer with the monocyte-telephone interactions mediated by CD4 that are necessary for the specific responses of the antigen. In addition, the crossing of the CD4 molecules by protein over can make the cell not respond to Antigenous stimulation. During the long asymptomatic period of HIV infection, the virus resides predominantly in a latent and chronically low-level state within lymph nodes Cells T4 and, to a lesser extent, within monocytes-macrophages. Mechanisms for which the virus stays in this relatively quiescent state is not clear. Similarly, little is known about the events that trigger activation. In vitro, a Number of factors have been identified as associated with activation of the expression of HIV. These factors include antigens, mitogens, cotransfection or coinfection of heterologous virus, and cytokines. HIV thought regulation by these factors implies the induction of cell proteins that It joins the HIV DNA-promoting region and increases its expression. Monocyte-macrophage is a target cell for both in vivo and in vivo HIV infection vitro. The infection of these cells can occur through the CD4 receptor or via Fagocitosis. In contrast to T4 cells, monocyte-macrophages appear to be resistant to cellular lysis. In addition, the virus can reproduce intracellularly in monocyto-macrophages, with virions that sprout in intracytoplasmic vesicles. Like result, viral antigens cannot be expressed on cellular surface, potentially allowing monocytes-macrophages to escape immunity surveillance and transportation infection to other organs systems, especially the lungs and brain. Although mechanisms by which HIV induces neuropsychiatric abnormalities are unknown, macrophrase is thought to play an important role. HIV infection in the brain appears to be largely restricted to macrophages, which may indirectly damage neuronal tissue freeing neurotoxic factors or factors that induce inflammation. HIV can also interfere with the union of neurotropic factors their neuron receptors. Another mechanism of HIV-induced neuropathology can involve autoimmune phenomena. Clearly, part of neurological abnormalities in HIV-induced disease is due to the wide range of opportunistic infections and tumors that afflict AIDS Patients. Although other retrovirus can cause cancers directly, HIV may not transform in vivo or in vitro cells. The multitude of tumors found in AIDS patients may result from widespread removal of immunos or induction factors that induce cell proliferation, as may be the case of Kaposi sarcoma. The proviral DNA of HIV has not been found in any of these tumors. Host Defenses A wide range of HIV immune responses have been observed at all stages of HIV infection. Antibodies for structural and regulatory proteins of HIV They usually appear several weeks to months after the initial infection. During the progression of HIV infection, antibody levels at the decline of central antigen p24, while antibodies to the protein of the envelope remain relatively constant. People infected with HIV produce antibodies that neutralize the virus, and these decrease in the last stages of AIDS. Antibodies that mediate dependent antibodies Cellular cytotoxicity (ADCC) has also been observed in the HIV/AIDS phase Patients. Cell-mediated responses to HIV include T-cell proliferation and cytotoxicity mediated by T cells and natural killer cells (NK). Interferon has been found in some AIDS patients, but the co-infection of opportunistic viruses may Be responsible. Since HIV is ultimately thought to cause a slow progressive and persistent infection in all infected individuals, the role of Immune responses to prevent HIV-induced disease are unclear. The emergence of the genetic variants of live HIV during the disease can be a way for HIV evade both humoral and cellular immune responses. EpidemiologyThe HIV-1 infection has been reported around the world both in development and developing countries. In the United States and other developed countries, HIV infection is predominantly found in homosexual and bisexual and intravenous men drug users. Hemophiliacs, transfusion receptors, sexual partners of infected People and babies born to infected mothers also have a high risk. In many parts of Africa and the Caribbean, HIV-1 is predominantly found in heterosexuals, transfusion receptors and babies born of infected mothers. HIV-2 is predominantly found in West Africa, Portugal and Brazil. Both HIV-1 and HIV-2 spread by sexual contact, exposure to contaminated blood or blood products, and perinatally of a mother infected with her offspring. That's it. estimated in 1990, between 800,000 and 1.3 million people The United States is infected with HIV. It is not known what proportion of these individuals will develop AIDS. The average incubation period is believed to be approximately 10 years. DiagnosisThe HIV infection is determined by the presence of HIV-specific antibodies, which usually occurs weeks after infection. HIV-related immunosorbent trial commonly used as the initial detection test, but the current approved by the FDA Trials can lose many early infected people. There are sporadic reports of individuals in which anti-HIV antibodies could not be detected for several years after the infection. A person is considered to be infected with HIV if he is positive test is confirmed by a positive Western block or a similar, more specific rehearsal. Diagnosis of HIV-2 infections requires specific HIV-2 tests. Detection of nucleic acid of the virus by the polymerase chain reaction can be used for diagnose HIV infection in negative antibodies. AIDS is diagnosed predominantly on the basis of opportunistic infections or indicative cancers of a T4 cell defect in the absence of a known cause Immunodeficiency. Neuropsychiatric manifestations are also indicative of AIDS. Although neurological complications may arise from opportunistic infections of the central nervous system, tumors and vascular problems, AIDS may occur in the absence of these conditions as HIV encephalopathy diagnosed Clinical findings of cognitive or motor dysfunction that interfere with the journal activities. AIDS can also be diagnosed in an HIV-infected individual weight loss base unexplained with chronic or chronic diarrhea weakness and fever, known as HIV waste syndrome. ControlStrategies for the control of HIV infection and disease are similar to those for VHT infection: therapy, education and public health, and vaccination. Therapeutic categories for HIV include antiviral therapies, immune modulators, and therapies to treat and prevent opportunistic diseases. server core analogous, are currently the only anti-HIV agents that have been proven Significantly improves survival in AIDS patients. Additional nucleoside analogs, as well as other potential antiviral agents, they are experiencing clinical trials. A combination of antiviral agents can be the most effective treatment for AIDS since it can allow the use of lower doses of drugs to minimize toxicity. Due to the persistence of HIV infection, permanent antiviral treatment will be It's probably necessary. In addition to antiviral agents, several Immunomodulator agents are subject to clinical trials. Regarding treatment of opportunistic infections, the use of aerosolized pentamidine has brought about a major advance in the prevention of pneumonia P carinii. From P carinii pneumonia is the most common cause of death in AIDS patients, aerosolized pentamidine can greatly improve survival in these Patients. Intensive educational efforts are needed to prevent sexually motivated HIV transmission sexual intercourse, intravenous drug use and blood exposure or blood products. Sexual abstinence, monogamous sexual intercourse with uninfected couples, use of condoms, and abstinence of intravenous drug use or clean use needles for HIV control through sexual intercourse and Intravenous drug use. HIV transmission can be prevented in health care workers and other medical environments through universal precautions (i.e. treating all blood as potentially infected with HIV). blood products are avoided by the detection of blood donated for anti-HIV antibodies and excluding high-risk donors from HIV infection. Women infected with HIV Advice should be given on the risk of 20% to 30% of the child perinatal transmission of HIV infection. An important obstacle to the development of an HIV vaccine is that the relationship of immunity to HIV infection is not understood. Lessons learned in the development of an animal retrovirus vaccine, feline leukemia virus, suggests that immunity to viral protein is crucial to prevention of infection. Efforts have therefore been made to develop HIV vaccines that use HIV HIV precursor glycoprotein (gpl60) or its external subunity (gpl20) Immunogens. These over subunit vaccines are being evaluated in phase 1 clinical trials of safety and immunogenicity in uninfected volunteers. In In addition, phase 1 testing is under way with inactive HIV preparations in symptomatic individuals infected with HIV. Although the usual use of a vaccine is to prevent infection with a microorganism, a vaccine against HIV is expected also prevent the development of AIDS in people already infected with HIV. References Views In this pageRelated information Similar products in PubMed Recent activityYour navigation activity is empty. The activity recording is off. , 8600 Rockville Pike, Bethesda MD, 20894 USA
Solved: Which Statement About DNA Replication Is CORRECT? ... | Chegg.com
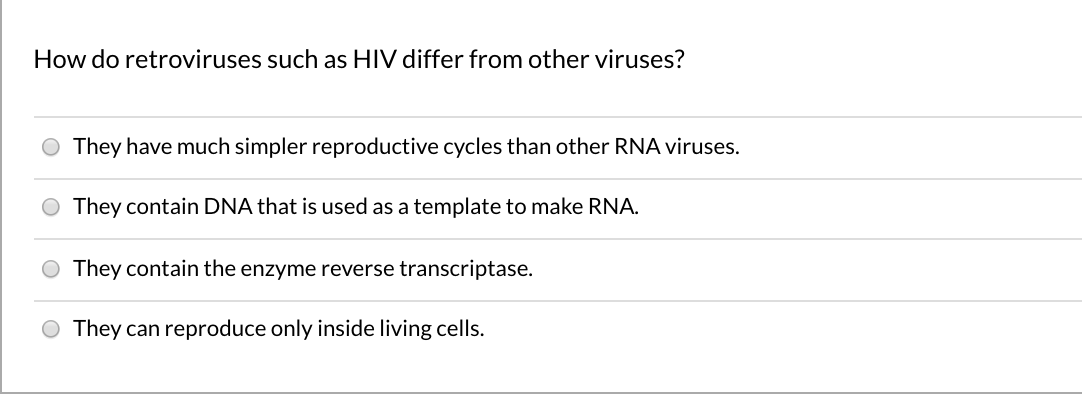
Retroviruses: Double-Stranded RNA Viruses | Boundless Microbiology

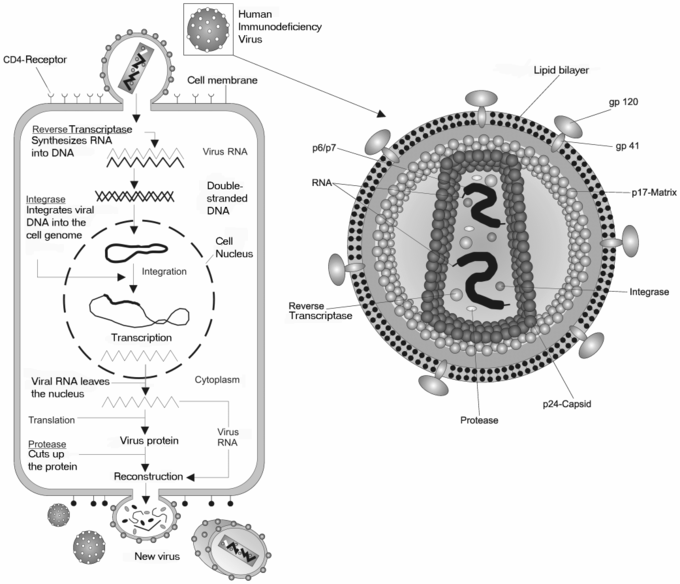
Molecular Biology and Pathogenesis of Retroviruses | IntechOpen

retrovirus | Definition, Examples, Diseases, Replication, & Facts | Britannica

Retroviridae - an overview | ScienceDirect Topics

Retroviruses: Double-Stranded RNA Viruses | Boundless Microbiology

Human Immunodeficiency Virus (HIV) Infection - Infections - Merck Manuals Consumer Version

Retrovirus - an overview | ScienceDirect Topics

Retrovirus: Definition, Life Cycle & Example - Video & Lesson Transcript | Study.com
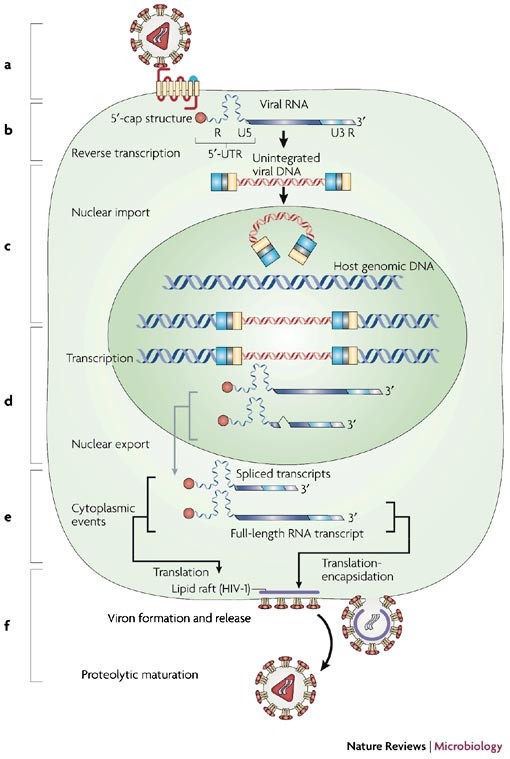
Translational control of retroviruses | Nature Reviews Microbiology
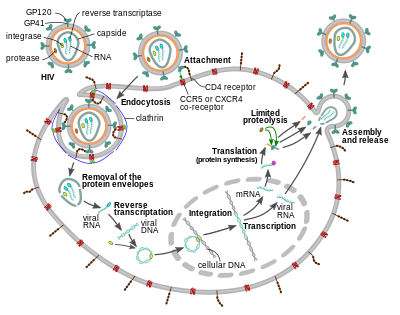
HIV - Wikipedia
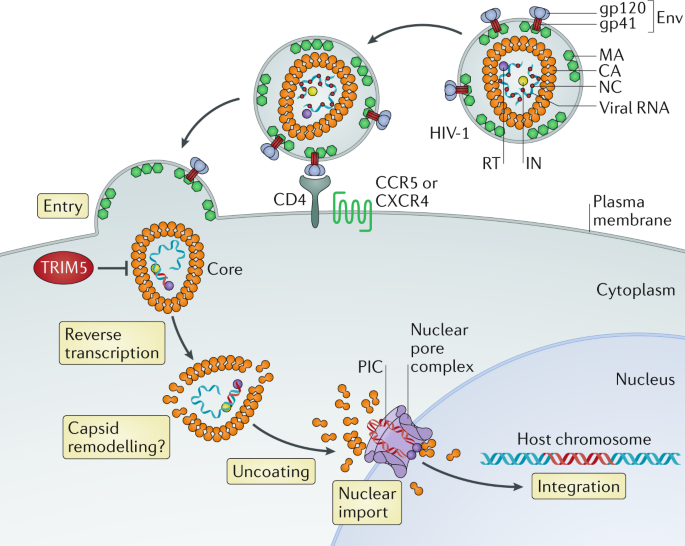
Restriction of HIV-1 and other retroviruses by TRIM5 | Nature Reviews Microbiology

Retroviruses (video) | Translation | Khan Academy
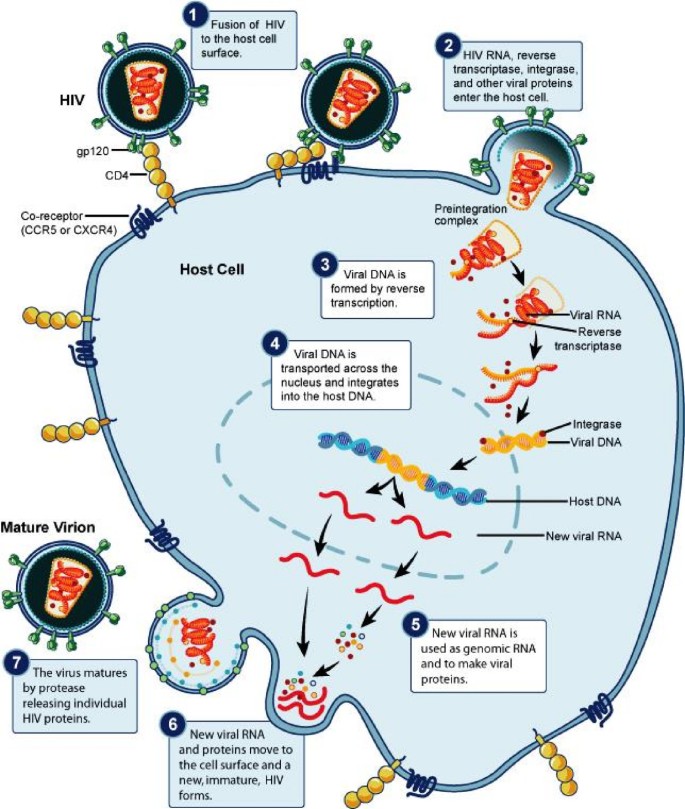
HIV is a retrovirus
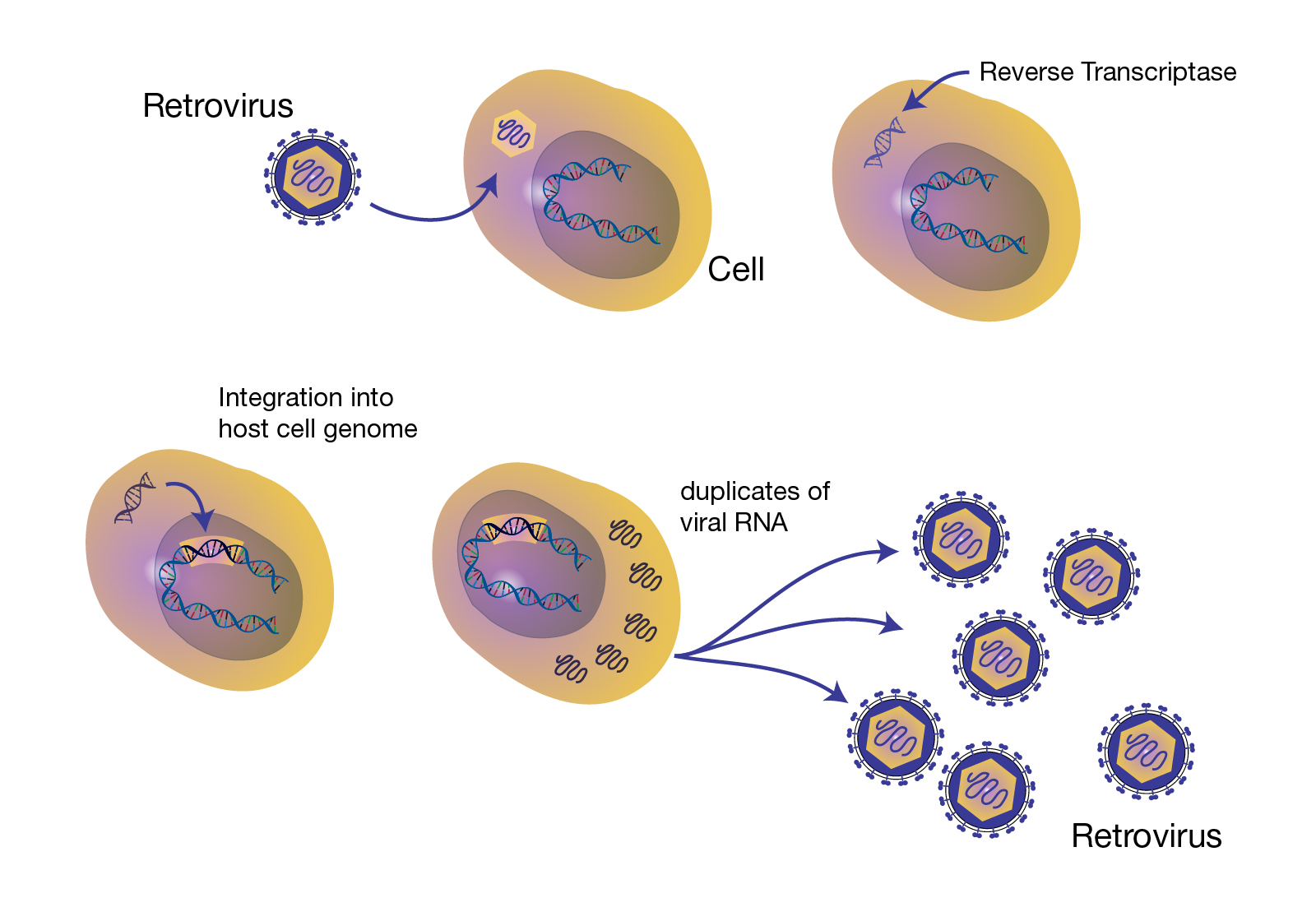
Retrovirus
On the general theory of the origins of retroviruses | Theoretical Biology and Medical Modelling | Full Text

Endogenous retrovirus - Wikipedia

Retroviruses are re-writing the koala genome and causing cancer

retrovirus | Definition, Examples, Diseases, Replication, & Facts | Britannica

Ancient Viruses Are Buried in Your DNA - The New York Times
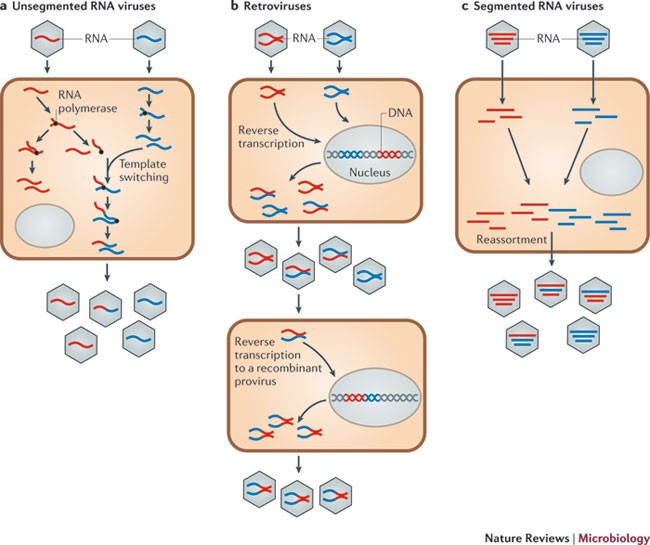
Why do RNA viruses recombine? | Nature Reviews Microbiology
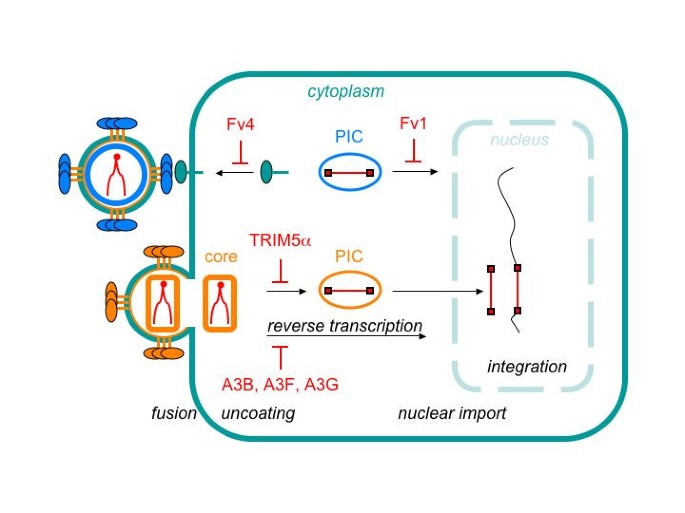
Intracellular immunity to HIV-1: newly defined retroviral battles inside infected cells | SpringerLink

Retrovirus Infection - an overview | ScienceDirect Topics

dna and rna Viruses

PDF) Intracellular immunity to HIV-1: Newly defined retroviral battles inside infected cells

Virus - AccessScience from McGraw-Hill Education
Wednesday, February 24, 2010

Viruses: How Are They Different from Bacteria?

Addgene: Retrovirus Guide

Biomolecules | Free Full-Text | HIV Vaccine Mystery and Viral Shell Disorder | HTML
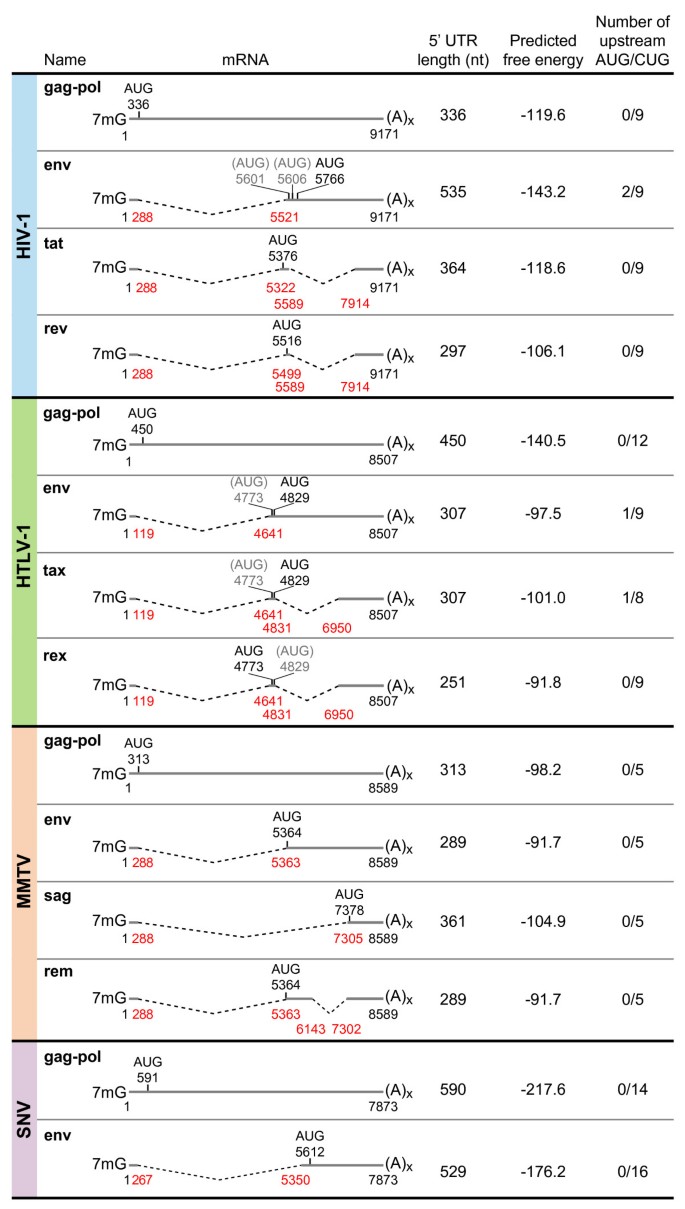
Mechanisms employed by retroviruses to exploit host factors for translational control of a complicated proteome | Retrovirology | Full Text

Biology+1001A+Practice+Oct+Test - StuDocu

Budding viral hijackers co-opt the endocytic machinery to make a getaway | SpringerLink

Human Immunodeficiency Virus (HIV) Infection - Infections - MSD Manual Consumer Version

What Is a Retrovirus? Comparison to Other Viruses, Examples, More
Curriculum Vitae PERSONAL INFORMATION Maja Sommerfelt Gronvold
PDF) A novel retroviral vector system to analyze expression from mRNA with retained introns using fluorescent proteins and flow cytometry

Can Viruses in the Genome Cause Disease? | The Scientist Magazine®
Modification of Human Immunodeficiency Virus Type 1 Reverse Transcriptase to Target Cells with Elevated Cellular dNTP Concentrat
Posting Komentar untuk "how do retroviruses such as hiv differ from other viruses?"